Lakes experience a chemical and hydrological transformation in the fall. This is thanks to one of water's unique properties: unlike most materials, it is most dense as a liquid. Water's maximum density occurs at 4 degrees Celsius (39.2 degrees Fahrenheit). It becomes less dense above and below this temperature. In lakes, cooling surface temperatures, aided by wind, cause the surface waters to become denser and sink, pushing the deeper cool water up and mixing it with the warmer waters above. This is known as the fall turnover.
Lake turnover is the seasonal mixing of the entire water column. For most Adirondack lakes deeper than about 20 feet, distinct, thermally stratified layers of water form during the summer. Warmer and less dense water floats on the top of cooler, denser water at the bottom. These layers prevent the lake from mixing and aerating. As summer transitions to fall, layering begins to weaken when outside temperatures cool. This allows the lake to mix when temperatures equalize throughout the water column. Eventually, the surface of the lake cools to 4 degrees Celsius. This causes the water at the surface to settle to the bottom, pushing the now relatively warmer water at the bottom back to the surface. This process continues until the surface water cools below 4 degrees Celsius, at which point it becomes less dense, and eventually freezes. If not for water's unique characteristic of decreasing in density as a solid, lakes would freeze from the bottom up, eventually freezing solid. In that case, little or nothing would survive in the lake. Most lakes and ponds don't completely freeze because the ice (and eventually snow) on the surface acts to insulate the water below.
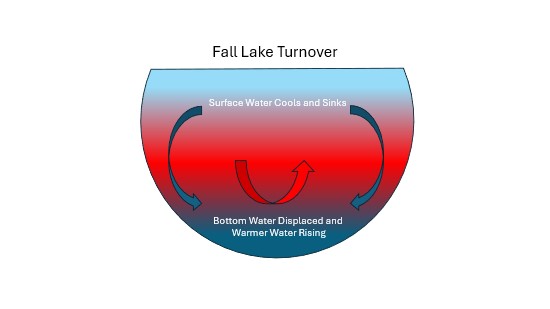
While lake turnover occurs in both the spring and the fall, the processes driving each differ. In many cases, fall lake turnover takes longer than spring lake turnover because in the spring, the warming surface water quickly reaches a density similar to the colder bottom water, leading to a more rapid mixing process. In fall, the cooling surface water takes longer to reach the same density as the deeper water, making the turnover slightly slower. The exact timing of both turnovers can vary depending on the specific lake and its climate conditions.
Along with the mixing and equilibration of water temperatures, it is these turnover periods that infuse and distribute oxygen throughout the entire water column. The redistribution of oxygen during turnover is critical to maintaining a healthy aquatic ecosystem. If oxygen is not replenished, the amount of viable habitat for fish and other aquatic organisms will drastically decline.
Support our water quality work for clean water and healthy streams. Give with confidence today!
Nutrients from lake sediments are also distributed during mixing. A telltale sign of lake turnover is a murky appearance to the lake, as sediments and nutrients from the lake bottom will become temporarily suspended throughout the water column, and the influx of phosphorus and nitrogen can give rise to agal blooms.

The redistribution of oxygen and nutrients in the spring and fall is vital to aquatic ecosystem health. Unfortunately, many midsized and deep lakes are experiencing longer periods of stratification during the summer due to lake browning and a warmer climate. This can lead to longer periods of deep-water anoxia, or the loss of dissolved oxygen. In anoxic conditions, nutrients such as phosphorus and nitrogen become more soluble, or readily dissolved in the water, and are released from the bottom sediments in higher concentrations. During turnover, this excess of nutrients can lead to harmful algal blooms (HABs), especially on warm calm days.

Murky water collected from a pond that recently experienced fall turnover.
The biannual cycle of lake turnover is essential in mitigating the negative impacts of low oxygen zones and providing suitable habitat for fish and aquatic organisms to thrive. Many lakes in our area have recently turned over or will do so soon. If you catch them at the right time, you may be able to see the evidence.
Story by Phil Snyder, Water Quality Research Manager.
Sign-up for our e-newsletter to get weekly updates on the latest stories from the Ausable Freshwater Center.


